Introduction
In the oil & gas industry, chemical systems are not just support functions — they are integral to production performance, asset integrity, and operational safety. Among these, Sodium Hydroxide (NaOH) — also known as caustic soda — plays a surprisingly important but often overlooked role.
Most people associate Sodium Hydroxide with soap manufacturing, paper processing, water treatment, or simple pH adjustment.
But in modern oilfield chemistry, NaOH contributes to:
drilling fluid formulation
crude oil refining
well stimulation jobs
demulsification & desalting units
enhanced oil recovery (EOR) systems
Despite being a basic commodity chemical, NaOH is tied to complex challenges when used in oilfield environments. It affects fluid rheology, interacts with polymers, influences corrosion behavior, and demands strict control due to its aggressive alkaline nature.
This blog breaks down — in a clear, easy-to-understand way — how Sodium Hydroxide fits into oilfield operations, its challenges, and the next-generation innovations that make its use safer, more efficient, and more predictable.
What exactly is Sodium Hydroxide? (Simple Breakdown)
Sodium Hydroxide (NaOH) = a strong, highly alkaline base.
Visually — in industry — it comes in 3 commercial forms:
Form | Typical Use |
Liquid 32–50% solution | Most common in refineries & drilling sites |
Solid flakes | Small batch or remote site supply |
Pellets/beads | Lab or controlled feed systems |
Chemical Behaviour:
extremely high pH (~13 to 14)
aggressively reacts with acids
absorbs CO₂ from air
dissolves organic deposits (asphaltenes, certain gums, etc.)
In oilfield context, NaOH is usually used diluted — between 0.5 to 2% — depending on the application.
Where Does NaOH Fit in Oil & Gas Applications?
Here are the six most relevant operational zones where Sodium Hydroxide is applied in petroleum workflows:
Application Zone | Purpose of NaOH |
Drilling Fluids | Raise alkalinity, control rheology, improve polymer hydration |
Mud Conditioning | Neutralize acidic contaminants, stabilize viscosity |
Well Stimulation | Used in certain surfactant + alkaline flooding blends |
Refinery Desalting | pH adjustment prior to desalter — reduces corrosion |
Demulsifier Support | Enhances demulsifier efficiency by adjusting interfacial tension |
Slop/Produced Water Treatment | Pre-treatment before coagulants in certain systems |
pH stabilizer
acidity neutralizer
polymer activator
Why This Topic Matters Today
Oilfield operators globally are chasing 3 goals:
Priority | Industry Pressure |
lower OPEX | chemical optimization & less waste |
safety | aggressive alkali = safety risk if mishandled |
sustainability | minimize caustic consumption & discharge |
So the way NaOH is used in 2025 is not the same as 10 years ago.
It’s no longer just “add caustic to adjust pH”.
Engineers now ask:
What polymers are incompatible with high pH?
What corrosion & stress cracking risks increase due to NaOH?
How much caustic can we safely use with metal surfaces present?
Is there a better way to deliver NaOH without spikes & shock reactions?
This is where challenges & innovations become critical.
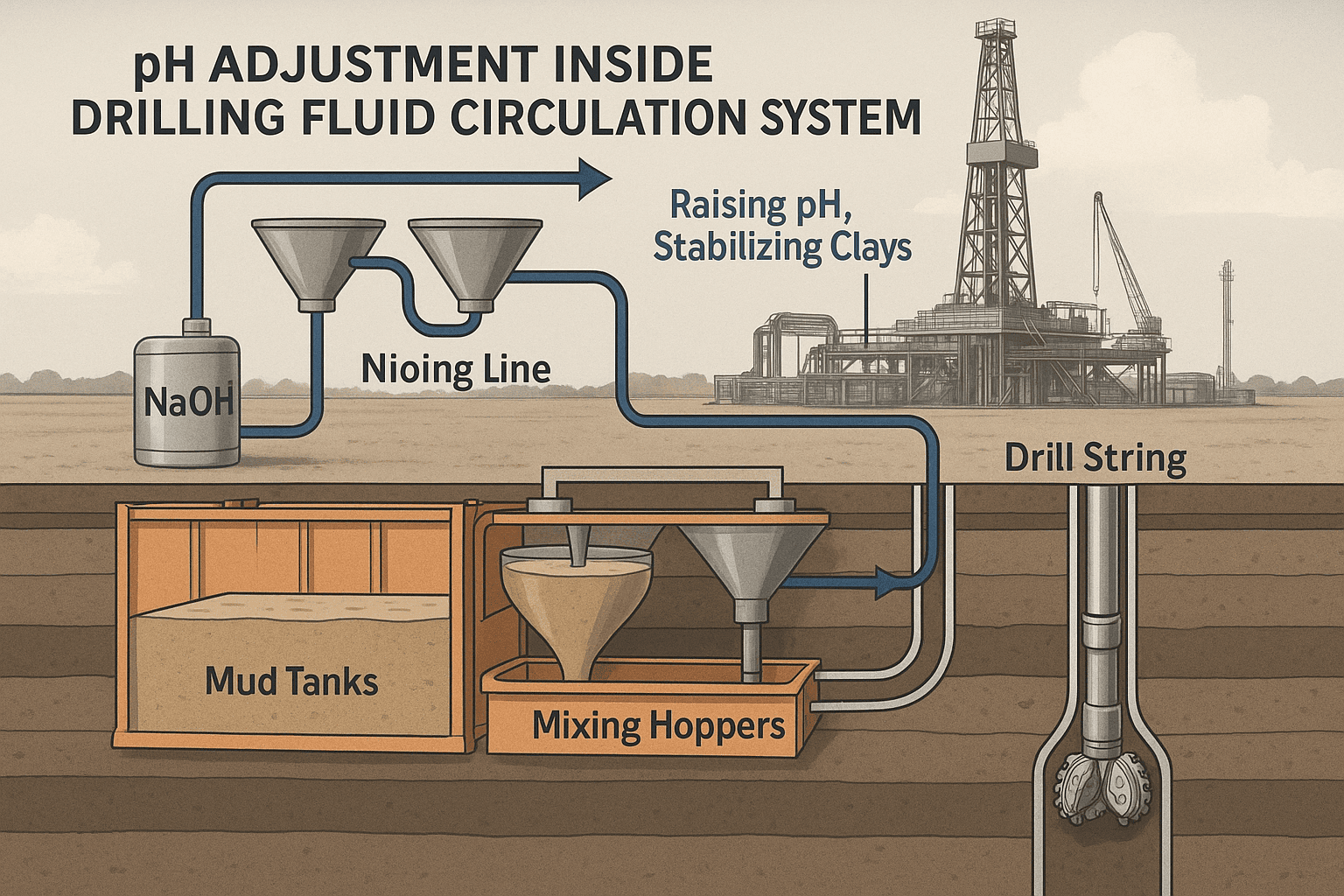
While Sodium Hydroxide is extremely useful in petroleum operations, it comes with a set of non-negotiable challenges. These issues are not “minor”—they can disrupt drilling fluid stability, damage metallurgy, or even trigger unsafe field conditions if not properly monitored.
Below are the most critical challenges that field engineers face when using NaOH.
1) Over-Alkalinity Can Break Fluid Rheology
This is probably the single most frequent mistake.
Most drilling fluids contain:
polymers (CMC, PAC, HEC)
biopolymers (Xanthan Gum, Guar)
lignosulfonates
surfactants
➡ These chemicals are extremely sensitive to pH.
If NaOH dosage overshoots even slightly, viscosity can jump dramatically or completely collapse depending on system chemistry.
Examples:
| pH Range | Risk Zone |
| < 10.0 | polymers under-hydrate, weak gels |
| 10.5 – 11.5 | optimum hydration window |
| > 12.0 | polymer degradation / rheology breakdown |
So NaOH must never be pumped blindly — it must be controlled precisely.
2) NaOH Aggressively Attacks Metals
Sodium hydroxide can corrode several metal alloys commonly used in field assets:
Asset Type | Common Material |
drilling pumps | carbon steel |
tubulars / tanks | mild steel |
refinery pipework | stainless variants |
High alkalinity accelerates:
caustic stress corrosion cracking (CSCC)
pitting
hydrogen embrittlement (in some cases)
This is why NaOH systems often require the addition of corrosion inhibitors to protect assets.
3) NaOH Absorbs CO₂ — Making pH Drift Unpredictable
NaOH reacts readily with carbon dioxide from air → forming sodium carbonate.
This is a chemical trap many new chemical engineers don’t notice.
Result: pH starts drifting downward without apparent chemical addition.
So a system that was adjusted to 11.2 in the morning may read 10.6 later — without any visible reason.
That small drift can ruin polymer hydration cycles, especially in reservoir fluid preparations.
4) Safety Hazards in Handling & Transfer
NaOH is highly caustic.
Challenges include:
burns on skin contact
eye & face flash risk during injection
strong exothermic reaction when diluted
This isn't a “lab injury” risk — this is real field severity.
Any splash incident in well intervention or mud mixing units can be serious.
5) Incompatibility with Certain Process Chemicals
NaOH can neutralize or deactivate certain production chemicals.
For example:
some surfactants lose efficiency at extreme pH
some biocides become unstable under high alkalinity
iron scale dissolvers can precipitate in alkaline conditions
This means NaOH must always be evaluated in a system — not as an isolated additive.
One chemical mismatch can cost millions due to:
downtime
non-productive time (NPT)
cleanup & re-treatment
6) Wastewater Discharge Impact
If high-pH waste streams are discharged — treatment units must neutralize them.
This adds extra steps:
Required Extra Treatment |
|
|
|
So uncontrolled NaOH use also increases wastewater treatment cost and chemical footprint.
Why These Challenges Matter
In the past decade, the industry has moved from chemical quantity-based thinking → toward chemical precision thinking.
Meaning:
Today, success depends not on how much chemical is added…
but on how precisely it is controlled and integrated into the system.
NaOH has become a symbol of that shift.
It is a simple chemical, yet one of the most technically sensitive in high-performance oilfield chemistry.
For decades, NaOH usage in oilfield operations was mostly manual and experience-driven.
A mud engineer or production chemist adjusted pH based on:
mud report sheets
dye indicator colour shift
basic titration kits
But today — the way we use NaOH in the oil & gas industry has changed dramatically.
The industry is moving toward precision alkalinity management, and NaOH is part of that transformation.
Below are the most notable innovations.
1) Smart Dosing Systems & Automated Injection Skids
In modern drilling & produced-water treatment facilities, NaOH is increasingly handled through:
PLC-based dosing skids
closed transfer chemical injection units
automated pump controllers
These systems maintain pH in tight, pre-programmed windows.
Benefits:
Advantage | Impact |
precise pH control | better polymer hydration |
reduced chemical overuse | expense savings |
lower operator exposure | improved safety |
consistent performance | fewer fluid upsets |
This shift reduces operator risk and protects fluid consistency across shifts and crew changes.
2) Digital pH Monitoring & AI-Based Predictive Adjustments
Some advanced operators now use:
real-time online pH metering
cloud-based dashboards
AI-driven dosing estimation
Instead of “test, react, add more” → systems now predict the required pH in advance based on:
mud system type
formation chloride loading
planned density change
cuttings composition
mineralogy models
This predictive, data-driven approach reduces NPT due to guesswork.
3) Pre-Activated NaOH Blended Packages (Multi-Chem Synergy)
Chemical formulators have started blending NaOH into multi-function additive packages.
Example:
PAC + polymer + NaOH = single-addition rheology stabilizing package
It reduces field mixing complexity.
Pre-blended packages:
reduce risk of incompatible sequencing
lower number of chemical transfer steps
eliminate on-site mixing errors
improve repeatability
This is especially valuable in:
Deepwater wells
ultra-high temperature reservoirs
tight timelines on rig operations
4) Safer Physical Formats — Less Powder, More Liquid
Traditionally — powdered NaOH pellets were common.
But powder form:
✔ is dusty
✔ attracts moisture
✔ causes air-borne skin/eye irritation risk
So the industry is shifting toward:
10–30% liquid sodium hydroxide solutions
pre-diluted solutions delivered in ISO tanks
mobile dosing totes with quick couplers
These changes protect crews and reduce field dilution risks.
5) Nano-Enabled Stabilizers for High-Salt and High-Temperature Systems
Experimental research & early commercialization (Middle East, U.S. Permian) shows that nanoparticle stabilizers can help NaOH-based chemistry stay stable even at extreme temperatures.
Example nano types being tested:
nano-silica
nano-alumina
nano-carbons
These “nano supports” prevent polymer breakdown under aggressive alkalinity.
This is especially relevant for:
HPHT wells
deep ultra-high temperature brine systems
6) Supply Chain Modernization — Bulk Delivery & Onsite Generation
Some refineries and offshore hubs now deploy membrane electrolysis units to generate NaOH onsite from brine.
Benefits:
benefit | impact |
removes shipping storage issues | safer |
zero packaging waste | ESG boost |
always fresh NaOH (less carbonate formation) | more stable pH |
This is one of the most overlooked innovations — but it is becoming more common globally.
Why These Innovations Matter
NaOH is no longer just a “simple caustic chemical.”
It is now integrated into a closed-loop, digitally monitored chemistry system.
The modern oilfield now focuses on:
chemical precision
predictive dosing
proactive material protection
ESG compliant operational efficiency
And NaOH — surprisingly — is becoming one of the clearest symbols of that transition.
Conclusion
Sodium hydroxide (NaOH) may look like a “simple” chemical at first glance — but in the oil & gas world, it behaves more like a strategic control lever for chemistry-driven performance.
Whether it’s drilling fluids, produced water polishing, polymer performance, or acid neutralization — NaOH supports some of the most critical chemical balances in the well lifecycle.
And as the industry continues to demand higher efficiency, lower environmental impact, and smarter dosing, NaOH is transforming from a manual additive into a precision-controlled industrial input — supported by automation, digital metering, pre-blended packages, and safer supply formats.
Oilfield chemistry is heading toward:
| The Past | The Now / Future |
| Manual trial-and-error dosing | AI-assisted dosing algorithms |
| Powder form caustic | pre-diluted liquid systems |
| Safety as an afterthought | safety designed into process steps |
| Chemistry stability issues | nano-reinforced stabilizing additives |
| Isolated chemical usage | chemical packages designed as synergy blocks |
NaOH is one of the chemicals witnessing this shift in real time.
Oilfield performance today is not only about what chemical you add —
but how precisely, safely, and intelligently you add it.
In that new environment — sodium hydroxide remains relevant, evolving, and vital.
Frequently Asked Questions (FAQs)
Q1. Is sodium hydroxide used only in drilling fluids?
No — besides drilling mud alkalinity control, NaOH is used in oilfield water treatment, refinery effluent pH control, polymer activation, scale removal, and acid neutralization.
Q2. Why is liquid NaOH preferred over solid pellets in many modern operations?
Liquid NaOH reduces dusting risk, improves handling safety, avoids moisture absorption, and supports automated metering pumps — ideal for closed-loop rigs or offshore platforms.
Q3. What is the biggest operational risk when using NaOH?
Operator exposure.
Skin contact, splashes into eyes, or inhaling caustic aerosols are major hazards, especially during manual dilution.
That’s why PPE, eyewash stations, and closed transfer systems are mandatory.
Q4. Can NaOH be replaced by other alkaline chemicals?
In some specific systems — potassium hydroxide (KOH) or amines may be alternatives.
But NaOH offers a unique balance of cost efficiency, strength, and availability — which is why it remains dominant.
Q5. Will NaOH usage increase or decrease in the future?
It will remain stable or increase slightly, but delivery formats will change — more automated injection, more pre-blended packages, and less manual handling.




