Introduction
In today’s evolving oil and gas industry, chemical management is the hidden backbone of safe and efficient hydrocarbon production. From drilling fluids to produced water treatment, every phase of an oilfield operation relies on carefully engineered chemical solutions that protect equipment, maintain flow efficiency, and ensure environmental compliance.
Among these essential chemicals, sodium hypochlorite (NaOCl) plays a particularly vital role. Commonly recognized as a powerful disinfectant and oxidizing agent, sodium hypochlorite has quietly become one of the most versatile and widely used treatment chemicals in both upstream and downstream oilfield applications.
Whether used to disinfect injection water, control microbial growth in pipelines, or treat produced water before discharge, its presence ensures the integrity of equipment and the safety of operational systems.Why Chemical Treatment Matters in Hydrocarbon Production
Oil and gas production involves handling large volumes of water—from drilling muds and completion fluids to produced and injected water. These water streams can introduce or support microbial activity, corrosion, and scaling—each capable of causing serious operational disruptions.
For instance:
- Microbial-induced corrosion (MIC) can deteriorate pipelines and storage tanks from the inside.
- Biofilm accumulation reduces flow rates and efficiency.
- Contaminated water can lead to formation damage or affect refining processes.
Thus, chemical treatment programs are not optional—they’re fundamental to oilfield reliability. Among the many treatment options available, sodium hypochlorite stands out for its strong oxidizing ability, cost-effectiveness, and ease of onsite generation.
Understanding Sodium Hypochlorite
Chemically, sodium hypochlorite (NaOCl) is a chlorine-based compound that functions as a strong oxidizing and disinfecting agent. It’s produced either by dissolving chlorine gas in sodium hydroxide or through electrolytic processes that generate it from brine (salt water).
In aqueous form, sodium hypochlorite produces hypochlorous acid (HOCl), a powerful oxidizer capable of neutralizing bacteria, viruses, organic contaminants, and sulfides—common culprits in oilfield fouling and corrosion.
Its dual action—oxidation and disinfection—makes it highly useful for:
- Microbial control: Eliminating sulfate-reducing bacteria (SRB) that cause hydrogen sulfide production.
- Odor removal: Oxidizing sulfides and organic matter responsible for unpleasant odors.
- Biofilm removal: Breaking down biological films that form on pipelines and equipment.
- Water disinfection: Ensuring the microbiological safety of injection and produced water.
Sodium Hypochlorite in the Oilfield Ecosystem
Oilfield operations—especially in hydrocarbon production—deal with complex fluid systems containing water, hydrocarbons, gases, and solids. Sodium hypochlorite finds application in multiple points across this chain:
Produced Water Treatment
Removes microbial contamination before disposal or reinjection.
Prevents anaerobic bacteria from producing hydrogen sulfide (H₂S).
Injection Water Disinfection
Ensures the injected water used in enhanced oil recovery (EOR) or pressure maintenance is microbiologically safe.
Reduces reservoir souring and formation plugging.
Pipeline and Storage Maintenance
Controls microbial corrosion and biofilm formation in pipelines, valves, and storage tanks.
Keeps system surfaces clean, ensuring uninterrupted flow and lower frictional losses.
Cooling Water Systems
Acts as a biocide to prevent slime and algae growth in refinery and petrochemical cooling towers.
In each of these systems, the key objective remains the same — to balance microbial control with material safety and operational efficiency.
Challenges in Handling and Application
Despite its benefits, sodium hypochlorite is a reactive and unstable compound, especially at high concentrations or elevated temperatures.
- It can decompose, releasing oxygen and chlorine gas if exposed to heat or light.
- It’s corrosive to certain metals, requiring careful material selection for tanks and piping.
- Overdosing can lead to residual chlorine issues in water discharge or downstream systems.
Setting the Stage for Safe & Sustainable Chemistry
As the oil and gas industry shifts toward sustainability and environmental stewardship, sodium hypochlorite’s role becomes even more important. Unlike some biocides and oxidizers, it is non-persistent, easy to neutralize, and decomposes into benign byproducts like salt and water under proper conditions.
In this context, it serves as a bridge between operational efficiency and environmental responsibility, embodying Trident’s vision of chemistry engineered for performance and safety.
Chemistry & Function in Oilfield Applications
Sodium Hypochlorite: The Chemistry That Powers Cleaner, Safer Oilfields
In hydrocarbon production, the efficiency of every process—from drilling to refining—depends on maintaining clean, stable, and microbially controlled fluid systems. Sodium hypochlorite (NaOCl), a simple yet powerful oxidizing agent, plays a critical role in achieving this balance. To appreciate its importance, it’s essential to understand how it works at the chemical level and how this chemistry translates to real-world oilfield performance.
The Chemistry Behind Sodium Hypochlorite
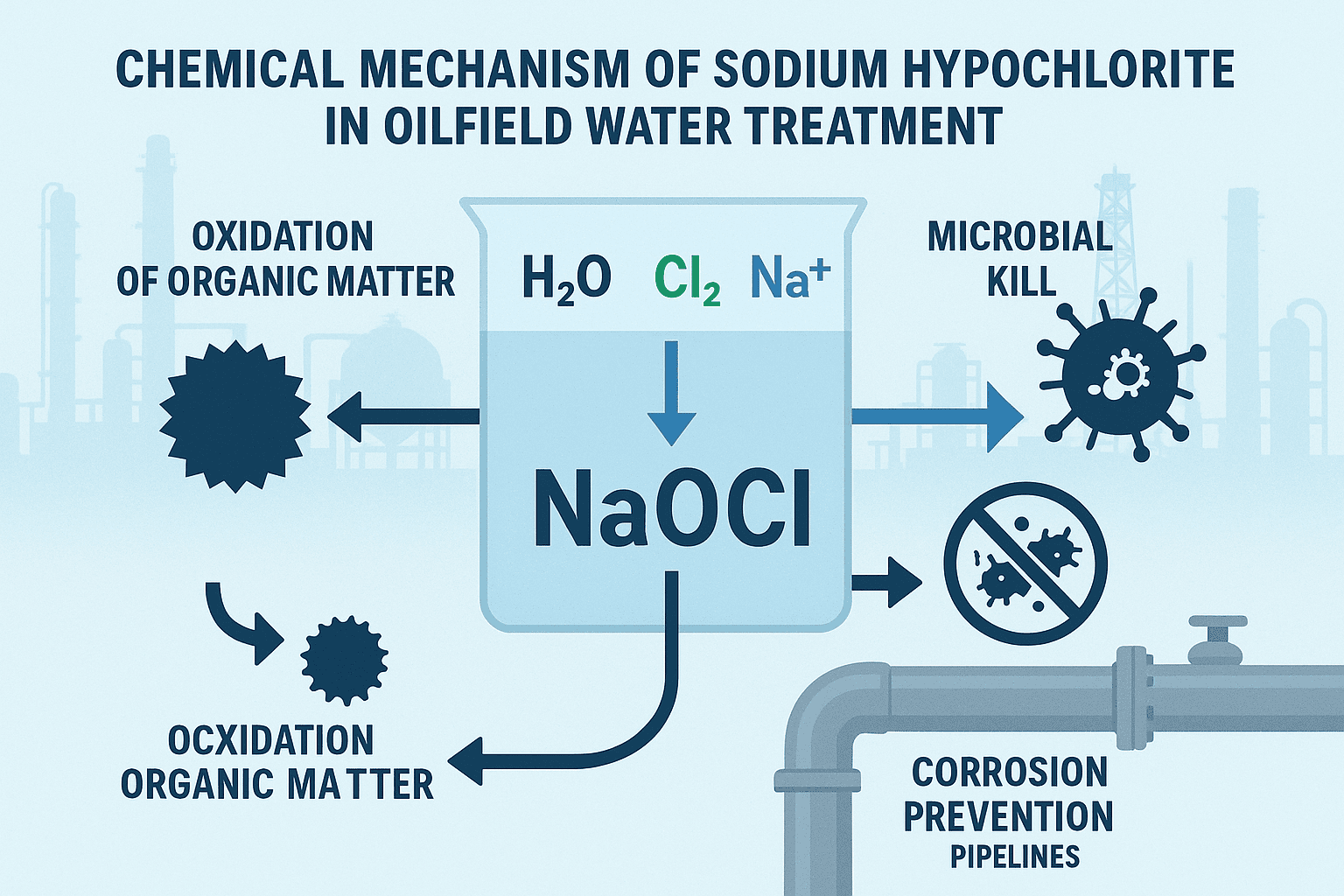
When dissolved in water, sodium hypochlorite forms a mixture of hypochlorous acid (HOCl) and hypochlorite ions (OCl⁻):
NaOCl+H2O⇌HOCl+Na++OH−
The relative proportion of these two species depends on the pH of the solution:
- At low pH (<7.5), hypochlorous acid (HOCl) dominates — it’s a stronger oxidizer and more effective disinfectant.
- At higher pH (>7.5), hypochlorite ion (OCl⁻) becomes dominant — less potent but more stable.
- HOCl quickly neutralizes microbes and organic contaminants.
- OCl⁻ maintains residual activity for long-term microbial control.
Microbial Control: The Frontline of Oilfield Hygiene
Microorganisms, particularly sulfate-reducing bacteria (SRB), are notorious in oilfield environments. These bacteria thrive in anaerobic conditions—such as pipelines, tanks, and downhole areas—producing hydrogen sulfide (H₂S), a toxic and corrosive gas.
Sodium hypochlorite combats this threat through oxidation and disinfection:
Destroys microbial cell walls, halting biological activity.
Oxidizes hydrogen sulfide (H₂S) into less harmful sulfate (SO₄²⁻).
Removes biofilms, preventing bacteria from attaching and proliferating on surfaces.
By maintaining microbial control, sodium hypochlorite helps avoid:
✔️ Pipeline and tank corrosion
✔️ Reduced flow due to slime buildup
✔️ Health hazards from toxic gas generation
✔️ Downtime caused by microbial plugging
This ensures continuous flow assurance and extended equipment life, making it indispensable for both upstream and downstream facilities.
Key Oilfield Applications of Sodium Hypochlorite
1. Produced Water Treatment
Produced water is one of the largest by-products of hydrocarbon production, often containing oil residues, organic matter, and microbes. Before disposal or reinjection, it must be disinfected to prevent reservoir contamination.
Sodium hypochlorite serves as an effective disinfectant, ensuring:
Elimination of microbial load.
Reduction of biological oxygen demand (BOD).
Oxidation of sulfides and residual hydrocarbons.
2. Injection Water and EOR Systems
Enhanced Oil Recovery (EOR) operations rely on injection water to maintain reservoir pressure or displace hydrocarbons. Any microbial contamination in this water can lead to:
- Reservoir souring (H₂S buildup)
- Formation damage
- Reduced permeability
3. Pipeline and Tank Cleaning
Pipelines and storage tanks carrying hydrocarbons are highly prone to biofilm and sludge accumulation.
When used in flushing and cleaning programs, sodium hypochlorite:
- Breaks down organic residues.
- Dissolves biofilm layers.
- Reduces odor and bacterial contamination.
It also supports pre-commissioning and maintenance cleaning of newly installed equipment, ensuring contaminant-free startup.
4. Cooling Water Systems
Refineries, petrochemical units, and LNG plants often operate large cooling water networks, which are breeding grounds for algae and slime. Sodium hypochlorite acts as a primary biocide, keeping these systems clean and ensuring:
- Improved heat exchange efficiency.
- Reduced maintenance frequency.
- Lower corrosion risk in cooling towers and heat exchangers.
5. Hydrogen Sulfide (H₂S) Control
One of the most critical safety challenges in hydrocarbon systems is H₂S gas, produced either naturally or by microbial action. Sodium hypochlorite oxidizes hydrogen sulfide to elemental sulfur or sulfate, significantly reducing its toxic and corrosive potential.
H2S+4NaOCl→Na2SO4+4NaCl+2H2O
This simple reaction demonstrates its dual advantage — safety enhancement and corrosion prevention in one process.
Compatibility with Other Oilfield Chemicals
While highly effective, sodium hypochlorite must be carefully integrated into treatment programs to avoid unwanted reactions.
For instance:
- It should not be mixed directly with amine-based corrosion inhibitors or surfactants, as it may oxidize them.
- Dosing should occur at separate injection points to ensure targeted action.
- It complements scale inhibitors and corrosion inhibitors, when properly sequenced, to create a balanced water treatment regime.
Handling and Material Considerations
Because of its oxidizing strength, sodium hypochlorite requires specific handling protocols:
- Materials: Use PVC, HDPE, or fiberglass tanks — avoid carbon steel.
- Storage: Protect from heat and sunlight to minimize decomposition.
- Safety: Personnel must use gloves, goggles, and protective clothing during handling.
- Dosing: Automated metering systems ensure consistent, safe addition to process lines.
Operational Efficiency & Safety Management
1. Integrating Sodium Hypochlorite into Oilfield Operations
The success of sodium hypochlorite (NaOCl) in hydrocarbon production lies not just in its chemistry but in how effectively it is integrated into the field’s operational design.
In oilfields, sodium hypochlorite is typically dosed into produced water treatment systems, injection lines, and cooling systems to control microbial growth, scaling, and corrosion.
Key integration points include:
- Produced Water Treatment: NaOCl oxidizes hydrogen sulfide (H₂S) and organic contaminants, preventing souring of water and improving reusability.
- Injection Wells: When injected into waterflood systems, it prevents biofilm buildup, ensuring consistent flow rates and preventing injectivity loss.
- Cooling Towers: It acts as a biocide, preventing algae and slime formation that can reduce heat exchange efficiency.
Properly integrating NaOCl reduces maintenance frequency, optimizes water quality, and extends asset life — directly linking to operational efficiency.
2. Monitoring & Dosing Control Systems
One of the critical factors in maximizing NaOCl’s performance is precise dosing control. Overdosing can lead to corrosion of pipelines and valves, while underdosing fails to control microbial contamination effectively.
Modern oilfields now use automated dosing systems equipped with sensors and PLC (Programmable Logic Controller) integration to monitor:
- Residual chlorine concentration
- Flow rate and pressure
- Temperature and pH levels
- Oxidation-reduction potential (ORP)
Through these automated setups, operators can ensure optimal biocidal activity with minimal waste — resulting in cost savings and enhanced safety.
For example, offshore platforms often use closed-loop chlorinationsystems that continuously adjust the sodium hypochlorite feed based on real-time microbial load or water quality parameters.3. Safety Considerations in Sodium Hypochlorite Handling
Despite its benefits, sodium hypochlorite requires careful handling due to its oxidizing and reactive nature.
Improper storage or mixing can result in hazardous situations such as chlorine gas release or exothermic reactions.
Key safety practices include:
Storage & Transportation
Store NaOCl in ventilated, UV-protected tanks made of compatible materials like HDPE or PVC.
Avoid metal containers, as hypochlorite reacts with iron and copper to form explosive chlorides.
Maintain temperature below 30°C to prevent decomposition into chlorine gas and sodium chlorate.
Mixing & Compatibility
Never mix sodium hypochlorite with acids, ammonia, or reducing agents.
Always dilute with clean water when preparing lower-concentration solutions.
Use backflow prevention devices in dosing lines to avoid contamination.
Personnel Protection
Operators should wear chemical-resistant gloves, goggles, face shields, and protective suits.
Eye wash and safety showers should be installed near the handling area.
Workers should be trained in chlorine exposure management and first aid procedures.
Spill & Leak Management
In case of minor spills, neutralize with sodium thiosulfate before rinsing.
For large spills, isolate the area and use containment dikes to prevent environmental release.
4. Environmental Safety & Waste Disposal
Though sodium hypochlorite degrades into harmless salts and oxygen, improper disposal can harm aquatic life and soil ecosystems.
Hence, oilfields follow strict neutralization protocols before discharging wastewater containing residual hypochlorite.
Common environmental practices include:
- Dechlorination using sodium bisulfite before wastewater discharge.
- On-site neutralization to maintain pH within environmental norms (6.5–8.5).
- Regular water quality testing for chlorine residuals to comply with pollution control standards.
5. Balancing Efficiency with Sustainability
As the oil and gas industry transitions toward greener operations, sodium hypochlorite continues to evolve.
Many producers now use on-site electrochlorination systems to generate NaOCl from seawater or brine, eliminating transportation hazards and chemical storage risks.
Advantages of on-site generation include:
- Reduced carbon footprint and logistics cost.
- Fresh, high-purity hypochlorite without degradation.
- Enhanced operator safety and sustainability.
This approach aligns with Trident’s sustainability philosophy — advancing efficiency without compromising environmental integrity.
Regulatory Compliance in Sodium Hypochlorite Use
Oilfield operations involving sodium hypochlorite must comply with strict local and international regulations to ensure worker safety, environmental protection, and process accountability.
A. Indian Regulations
In India, sodium hypochlorite use in industrial sectors, including hydrocarbon production, is governed by multiple standards and regulatory bodies such as:
Central Pollution Control Board (CPCB): Ensures that discharge and effluent levels comply with water and air pollution norms.
Occupational Safety, Health and Working Conditions Code (2020): Lays down chemical handling and safety training requirements.
Petroleum and Explosives Safety Organisation (PESO): Regulates chemical storage, labeling, and transportation safety for hazardous materials.
Factories Act, 1948: Mandates provision of protective gear, ventilation, and safety signage in chemical handling zones.
For offshore or joint-venture operations, compliance with Oil Industry Safety Directorate (OISD) guidelines is critical. OISD-STD-118, for instance, provides detailed norms for chemical storage, fire control, and environmental risk management in refineries and oilfields.
B. International Frameworks
Globally, sodium hypochlorite handling and discharge are regulated by:
OSHA (Occupational Safety and Health Administration, USA) – chemical labeling, worker exposure limits, and emergency procedures.
EPA (Environmental Protection Agency) – wastewater discharge and environmental risk assessments.
REACH & CLP (European Union) – registration, evaluation, and safe classification of chemicals.
IMO (International Maritime Organization) – specific to offshore and marine operations involving hypochlorite-based disinfection or corrosion control.
Meeting these compliance benchmarks enhances credibility and global acceptance of operations, especially for export-oriented oilfield companies.
Documentation and Safety Auditing
Every sodium hypochlorite handling site must maintain clear and updated documentation, including:
Material Safety Data Sheets (MSDS)
Chemical storage and inventory logs
Safety inspection and maintenance reports
Incident and exposure records
Environmental monitoring data
Regular internal audits and third-party assessments ensure all operational safety and environmental standards are met.
Companies like Trident often help clients develop custom compliance protocols — integrating documentation, digital monitoring, and emergency response frameworks for full-spectrum safety governance.
Future Trends: Towards Smarter and Greener Applications
The use of sodium hypochlorite in hydrocarbon production is evolving rapidly alongside advancements in digital monitoring, process automation, and green chemistry.
A. On-Site Electrochlorination Systems
One of the fastest-growing trends is on-site sodium hypochlorite generation, especially for offshore and remote oilfields.
These systems electrolyze seawater or brine, producing a stable hypochlorite solution on demand.
Benefits include:
No need to transport or store concentrated chemicals.
Reduced decomposition and chlorine gas hazards.
Lower lifecycle cost and carbon emissions.
B. Digital Control and Predictive Analytics
Next-generation control systems use IoT-enabled sensors and predictive models to automatically adjust sodium hypochlorite dosing based on real-time microbial activity, flow data, and temperature readings.
This reduces manual intervention, optimizes consumption, and prevents over-treatment.
C. Green Chemistry and Eco-Compatible Alternatives
Researchers are developing eco-friendly stabilizers that extend NaOCl shelf life without generating harmful by-products.
Additionally, biodegradable oxidants are being tested to complement sodium hypochlorite, providing safer discharge and minimal impact on marine ecosystems.
Together, these innovations signal a shift toward smart, sustainable, and self-regulating oilfield operations.
The Role of Trident in Advancing Safe Chemical Practices
Trident is not just a supplier of oilfield chemicals — it’s a strategic partner helping energy producers implement safe, efficient, and regulatory-compliant chemical programs.
Through its custom sodium hypochlorite formulations, technical support, and compliance expertise, Trident ensures:
Enhanced process efficiency and uptime.
Safe chemical integration across field operations.
Full adherence to environmental and industrial safety laws.
By balancing chemistry with responsibility, Trident stands at the forefront of the next generation of oilfield chemical innovation.
Conclusion
Sodium hypochlorite remains one of the most versatile and effective agents in hydrocarbon production — controlling microbial growth, preventing corrosion, and enhancing water quality throughout the process cycle.
When applied with precision dosing, rigorous safety measures, and strong regulatory compliance, it delivers unmatched operational efficiency while maintaining environmental integrity.
As the oil and gas industry advances toward digital, sustainable, and low-carbon operations, the role of sodium hypochlorite — and companies like Trident — will only become more central in driving safe, efficient, and future-ready production systems.
Frequently Asked Questions (FAQs)
1. Why is sodium hypochlorite preferred over other oxidizing agents in oilfield operations?
Because it’s cost-effective, easy to handle, and provides broad-spectrum disinfection and oxidation, making it ideal for large-scale water treatment in oilfields.
2. How does sodium hypochlorite control corrosion in hydrocarbon systems?
By eliminating sulfate-reducing bacteria and oxidizing organic matter, it prevents the microbial activity that often initiates under-deposit corrosion and pitting.
3. Can sodium hypochlorite be used safely in offshore platforms?
Yes. With proper storage, ventilation, and on-site generation systems, sodium hypochlorite is a safe and practical choice for offshore disinfection and corrosion control
4. What are the main environmental precautions when using sodium hypochlorite?
Residual chlorine should be neutralized before wastewater discharge, and operators must monitor effluent pH and chlorine levels to comply with pollution control standards.
5. How does Trident ensure safety in sodium hypochlorite applications?
Trident offers complete chemical management support — from on-site audits and safe handling training to automated dosing solutions and regulatory documentation.




